INDICATIONS:
KERENDIA (finerenone) is indicated to reduce the risk of:
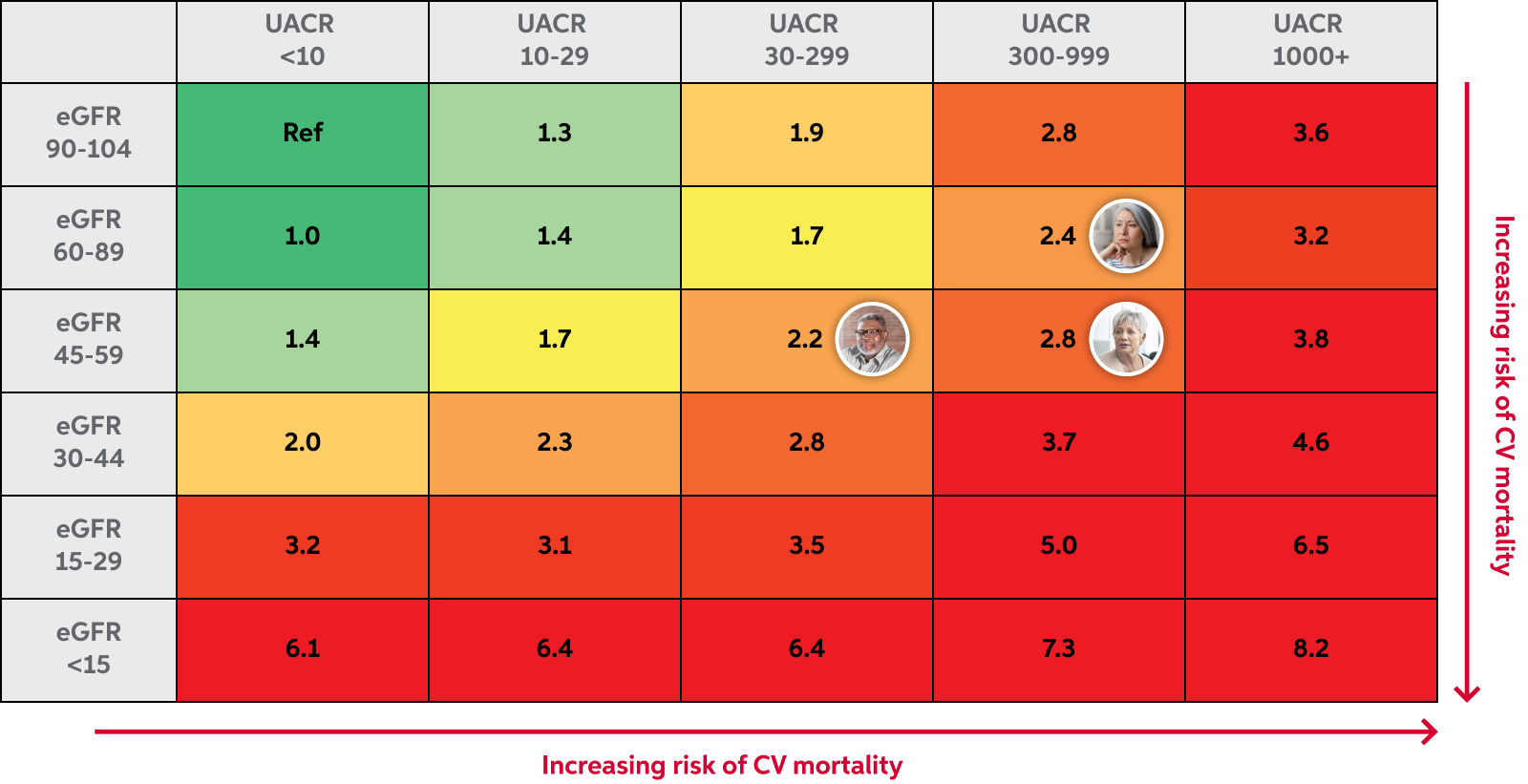
- sustained estimated glomerular filtration rate (eGFR) decline, end-stage kidney disease, cardiovascular death, non-fatal myocardial infarction, and hospitalization for heart failure in adult patients with chronic kidney disease (CKD) associated with type 2 diabetes (T2D) (10mg, 20mg tablets)
- cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in adult patients with heart failure with left ventricular ejection fraction (HF LVEF) ≥40% (10mg, 20mg, 40mg tablets)
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS:
- Hypersensitivity to any component of this product
- Concomitant use with strong CYP3A4 inhibitors
- Patients with adrenal insufficiency
WARNINGS AND PRECAUTIONS:
Hyperkalemia: KERENDIA can cause hyperkalemia. The risk for developing hyperkalemia increases with decreasing kidney function and is greater in patients with higher baseline potassium levels or other risk factors for hyperkalemia
Measure serum potassium and eGFR in all patients before initiation of treatment with KERENDIA and dose accordingly. Do not initiate KERENDIA if serum potassium is >5 mEq/L. Measure serum potassium periodically during treatment with KERENDIA and adjust dose accordingly. More frequent monitoring may be necessary for patients at risk for hyperkalemia, including those on concomitant medications that impair potassium excretion or increase serum potassium.
Worsening of Renal Function in Patients with Heart Failure: KERENDIA can cause worsening of renal function in patients with heart failure. Rarely, severe events associated with worsening renal function, including events requiring hospitalization, have been observed
Measure eGFR in all patients before initiation of treatment or with dose titration of KERENDIA and dose accordingly. Initiation of KERENDIA in patients with heart failure and an eGFR <25 mL/min/1.73 m2 is not recommended. Measure eGFR periodically during maintenance treatment with KERENDIA in patients with heart failure. Consider delaying up-titration or interrupting treatment with KERENDIA in patients who develop clinically significant worsening of renal function
MOST COMMON ADVERSE REACTIONS:
- CKD associated with T2D: From the pooled data of FIDELIO-DKD and FIGARO-DKD, the adverse reactions reported in ≥1% of patients on KERENDIA and more frequently than placebo were hyperkalemia (14% vs 6.9%), hypotension (4.6% vs 3%), and hyponatremia (1.3% vs 0.7%).
- HF LVEF ≥40%: From FINEARTS-HF, the adverse reactions reported in ≥1% of patients on KERENDIA and more frequently than placebo were hyperkalemia (9.7% vs 4.2%), hypotension (7.6% vs 4.7%), and hyponatremia (1.9% vs 0.9%). Events related to worsening renal function were reported more frequently in the KERENDIA group (18%) compared with placebo (12%).
DRUG INTERACTIONS:
- Strong CYP3A4 Inhibitors: Concomitant use of KERENDIA with strong CYP3A4 inhibitors is contraindicated. Avoid concomitant intake of grapefruit or grapefruit juice.
- Moderate and Weak CYP3A4 Inhibitors: Monitor serum potassium during drug initiation or dosage adjustment of either KERENDIA or the moderate or weak CYP3A4 inhibitor, and adjust KERENDIA dosage as appropriate.
- Strong and Moderate CYP3A4 Inducers: Avoid concomitant use of KERENDIA with strong or moderate CYP3A4 inducers.
- Sensitive CYP2C8 Substrates at KERENDIA 40mg: Monitor patients more frequently for adverse reactions caused by sensitive CYP2C8 substrates if KERENDIA 40mg is co-administered with such substrates, since minimal concentration changes may lead to serious adverse reactions.
USE IN SPECIFIC POPULATIONS:
- Lactation: Avoid breastfeeding during treatment with KERENDIA and for 1 day after treatment.
- Hepatic Impairment: Avoid use of KERENDIA in patients with severe hepatic impairment (Child Pugh C) and consider additional serum potassium monitoring with moderate hepatic impairment (Child Pugh B).